IZMIT, Turkey--(BUSINESS WIRE)--Jul. 16, 2018--
Masimo (NASDAQ:
MASI) announced today the findings of a recently published study in
which researchers at Kocaeli University in Turkey compared the
performance of conventional fluid management (CFM) to goal-directed
fluid management (GDFM) using Masimo PVi® (pleth variability
index, measured noninvasively and continuously using SET®
pulse oximetry sensors) in patients undergoing elective colorectal
surgery. The primary points of comparison were the amount of
crystalloids administered and blood lactate and serum creatinine levels
during the intraoperative period.1
This press release features multimedia. View the full release here:
https://www.businesswire.com/news/home/20180715005048/en/
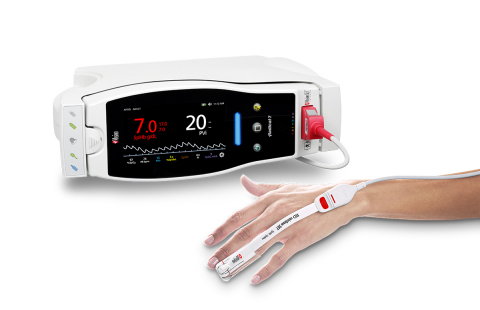
Masimo Radical-7® with PVi®, SpHb®, and RD rainbow SET™ Sensor (Photo: Business Wire)
In the study, Dr. Cesur and colleagues, noting the importance of
intraoperative fluid management in terms of postoperative organ
perfusion and complications, sought to compare the effects of CFM
(guided by clinical assessment and heart rate, arterial blood pressure,
and invasively measured central venous pressure) with GDFM (guided by
clinical assessment and noninvasive Masimo PVi monitoring). They
enrolled 70 ASA I-II adult patients undergoing elective colorectal tumor
surgery, who were divided randomly into CFM and GDFM groups. PVi was
measured using a Masimo Radical-7® Pulse CO-Oximeter®
with software version 7.0.3.3 and SET® sensors. In the CFM
group, an NaCl solution was administered at the rate of 4-8 ml/kg/h;
when mean arterial pressure (MAP) fell below 65 mmHg or 30% baseline
MAP, the speed of infusion was increased, colloid was initiated, and
ephedrine was administered. In the GDFM group, the same solution was
administered at the rate of 2 ml/kg/h; if PVi rose above 13% for more
than 5 minutes, colloid and then ephedrine were administered. Treatments
in both groups were continued until values were restored to each
protocol’s pre-treatment threshold.
The researchers found that intraoperative crystalloid administration,
urine output, and end-surgery fluid balance were significantly lower in
the GDFM (PVi) group:
| Characteristic |
|
|
Median value (25-75 percentile values): CFM group |
|
|
|
Median value (25-75 percentile values): GDFM (PVi) group |
|
|
|
P-value |
|
Intraoperative Crystalloid administration
|
|
|
1946 ml (1500-2500 ml)
|
|
|
|
900 ml (800-1060 ml)
|
|
|
|
<0.001
|
|
Urine output
|
|
|
400 ml (250-600 ml)
|
|
|
|
300 ml (200-400 ml)
|
|
|
|
0.018
|
|
End-surgery fluid balance
|
|
|
1400 ml (960-2250 ml)
|
|
|
|
620 ml (410-1000 ml)
|
|
|
|
<0.001
|
|
|
|
|
|
|
|
|
|
|
|
|
Durations of anesthesia and of surgery, as well as the amounts of
intraoperative bleeding and administered colloid, were similar. The
length of hospital stay was also found to be similar between the two
groups.
The researchers noted that a limitation of the study was that they chose
the number of subjects based on the needs of their primary objective,
the comparison of intraoperative fluid volume between the two protocols,
so they may not have evaluated enough subjects to effectively compare
secondary outcomes such as length of stay: “We determined the primary
goal of this study as the amount of intraoperative fluid volume and
established 35 patients were needed for each group; if postoperative
complications, the length of hospital stay[, were] determined as the
primary goal, perhaps our numbers [in] each group [w]ould be different.”
@MasimoInnovates |
#Masimo
Reference
-
Cesur S, Cardakozu T, Alparslan K, Turkyilmaz N, and Yavuz O.
Comparison of conventional fluid management with PVI-based
goal-directed fluid management in elective colorectal surgery. J
Clin Mon.14 June 2018. https://doi.org/10.1007/s10877-018-0163-y
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive
monitoring technologies. Our mission is to improve patient outcomes and
reduce the cost of care. In 1995, the company debuted Masimo SET®
Measure-through Motion and Low Perfusion™ pulse oximetry, which has been
shown in multiple studies to significantly reduce false alarms and
accurately monitor for true alarms. Masimo SET® has also been
shown to help clinicians reduce severe retinopathy of prematurity in
neonates,1 improve CCHD screening in newborns,2
and, when used for continuous monitoring with Masimo Patient SafetyNet™*
in post-surgical wards, reduce rapid response activations and costs.3,4,5
Masimo SET® is estimated to be used on more than 100 million
patients in leading hospitals and other healthcare settings around the
world,6 and is the primary pulse oximetry at 17 of the top 20
hospitals listed in the 2017-18 U.S. News and World Report Best
Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow®
Pulse CO-Oximetry technology, allowing noninvasive and continuous
monitoring of blood constituents that previously could only be measured
invasively, including total hemoglobin (SpHb®), oxygen
content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin
(SpMet®), Pleth Variability Index (PVi®), and more
recently, Oxygen Reserve Index (ORi™), in addition to SpO2,
pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®,
an intuitive patient monitoring and connectivity platform with the
Masimo Open Connect® (MOC-9®) interface, enabling
other companies to augment Root with new features and measurement
capabilities. Masimo is also taking an active leadership role in mHealth
with products such as the Radius-7® wearable patient monitor,
iSpO2® pulse oximeter for smartphones, and the
MightySat™ fingertip pulse oximeter. Additional information about Masimo
and its products may be found at www.masimo.com.
Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale
in the United States.
*The use of the trademark Patient SafetyNet is under license from
University HealthSystem Consortium.
References
-
Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm
Infants through Changes in Clinical Practice and SpO2
Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
-
de-Wahl Granelli A et al. Impact of pulse oximetry screening on the
detection of duct dependent congenital heart disease: a Swedish
prospective screening study in 39,821 newborns. BMJ. 2009;Jan
8;338.
-
Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue
Events and Intensive Care Unit Transfers: A Before-And-After
Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
-
Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia
Patient Safety Foundation Newsletter. Spring-Summer 2012.
-
McGrath SP et al. Surveillance Monitoring Management for General Care
Units: Strategy, Design, and Implementation. The Joint Commission
Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
-
Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in
Section 27A of the Securities Act of 1933 and Section 21E of the
Securities Exchange Act of 1934, in connection with the Private
Securities Litigation Reform Act of 1995. These forward-looking
statements include, among others, statements regarding the potential
effectiveness of Masimo PVi®. These forward-looking
statements are based on current expectations about future events
affecting us and are subject to risks and uncertainties, all of which
are difficult to predict and many of which are beyond our control and
could cause our actual results to differ materially and adversely from
those expressed in our forward-looking statements as a result of various
risk factors, including, but not limited to: risks related to our
assumptions regarding the repeatability of clinical results; risks
related to our belief that Masimo's unique noninvasive measurement
technologies, including Masimo PVi, contribute to positive clinical
outcomes and patient safety; risks related to our belief that Masimo
noninvasive medical breakthroughs provide cost-effective solutions and
unique advantages; as well as other factors discussed in the "Risk
Factors" section of our most recent reports filed with the Securities
and Exchange Commission ("SEC"), which may be obtained for free at the
SEC's website at www.sec.gov.
Although we believe that the expectations reflected in our
forward-looking statements are reasonable, we do not know whether our
expectations will prove correct. All forward-looking statements included
in this press release are expressly qualified in their entirety by the
foregoing cautionary statements. You are cautioned not to place undue
reliance on these forward-looking statements, which speak only as of
today's date. We do not undertake any obligation to update, amend or
clarify these statements or the "Risk Factors" contained in our most
recent reports filed with the SEC, whether as a result of new
information, future events or otherwise, except as may be required under
the applicable securities laws.

View source version on businesswire.com: https://www.businesswire.com/news/home/20180715005048/en/
Source: Masimo
Masimo
Evan Lamb, 949-396-3376
[email protected]