SedLine, Which Helps Clinicians Improve Anesthetic Management,
Receives CE Mark
NEUCHATEL, Switzerland--(BUSINESS WIRE)--May 26, 2016--
Masimo
(NASDAQ: MASI) announced today the CE mark and scheduled full market
release of Next Generation SedLine® Brain Function Monitoring
technology at the European Society of Anaesthesiology’s (ESA) 2016
Euroanaesthesia Congress in London, England.
This Smart News Release features multimedia. View the full release here:
http://www.businesswire.com/news/home/20160526005720/en/
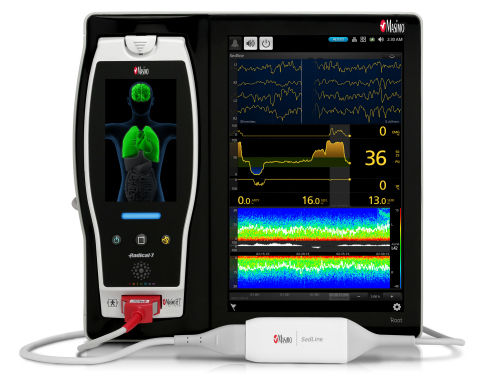
Root with Next Generation SedLine (Photo: Business Wire)
Next Generation SedLine enhances how Masimo’s processed EEG parameter,
the Patient State Index (PSI), responds in challenging situations,
addressing many of the concerns raised with quantitative EEG while
bolstering brain function monitoring’s support of anesthetic management.
Next Generation PSI uses Masimo’s breakthrough Parallel Engines and
Adaptive Signal Processing technology and provides the following
enhancements:
-
Less susceptibility to electromyography (EMG) interference by
extracting a clearer EEG signal even in the presence of EMG
-
Improved PSI performance in low power EEG cases
These improvements build upon the existing benefits of SedLine
technology:
-
Four simultaneous EEG leads to enable continuous assessment of both
sides of the brain
-
Density Spectral Array (DSA) offers easy-to-interpret, high-resolution
display of bi-hemispheric activity
-
Multiple screen views expand information while enabling customization
in the OR and ICU
-
Electrocautery resistance
“We are delighted to unveil Next Generation SedLine,” said Joe Kiani,
Founder and CEO of Masimo. “Next Generation PSI takes advantage of
Masimo’s signal processing prowess and promises to do for brain function
monitoring what SET did for pulse oximetry.”
Next Generation SedLine has not received FDA 510(k) clearance and is not
currently available for sale in the United States.
@MasimoInnovates
| #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive
monitoring technologies. Our mission is to improve patient outcomes and
reduce the cost of care by taking noninvasive monitoring to new sites
and applications. In 1995, the company debuted Masimo SET®
Measure-through Motion and Low Perfusion™ pulse oximetry, which has been
shown in multiple studies to significantly reduce false alarms and
accurately monitor for true alarms. Masimo SET® is estimated to be used
on more than 100 million patients in leading hospitals and other
healthcare settings around the world. In 2005, Masimo introduced
rainbow® Pulse CO-Oximetry technology, allowing noninvasive and
continuous monitoring of blood constituents that previously could only
be measured invasively, including total hemoglobin (SpHb®), oxygen
content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and
more recently, Pleth Variability Index (PVI®) and Oxygen Reserve Index
(ORI™), in addition to SpO2, pulse rate, and perfusion index
(PI). In 2014, Masimo introduced Root®, an intuitive patient monitoring
and connectivity platform with the Masimo Open Connect (MOC-9)
interface. Masimo is also taking an active leadership role in mHealth
with products such as the Radius-7™ wearable patient monitor and the
MightySat™ fingertip pulse oximeter. Additional information about Masimo
and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in
Section 27A of the Securities Act of 1933 and Section 21E of the
Securities Exchange Act of 1934, in connection with the Private
Securities Litigation Reform Act of 1995. These forward-looking
statements include, among others, statements regarding the potential
effectiveness of Masimo SedLine®. These forward-looking
statements are based on current expectations about future events
affecting us and are subject to risks and uncertainties, all of which
are difficult to predict and many of which are beyond our control and
could cause our actual results to differ materially and adversely from
those expressed in our forward-looking statements as a result of various
risk factors, including, but not limited to: risks related to our
assumptions regarding the repeatability of clinical results; risks
related to our belief that Masimo's unique noninvasive measurement
technologies, including Masimo SedLine®, contribute to
positive clinical outcomes and patient safety; risks related to our
belief that Masimo noninvasive medical breakthroughs provide
cost-effective solutions with comparable accuracy and unique advantages,
including: immediate and continuous results that enable earlier
treatment without causing invasive trauma in all patients and in every
clinical situation; as well as other factors discussed in the "Risk
Factors" section of our most recent reports filed with the Securities
and Exchange Commission ("SEC"), which may be obtained for free at the
SEC's website at www.sec.gov.
Although we believe that the expectations reflected in our
forward-looking statements are reasonable, we do not know whether our
expectations will prove correct. All forward-looking statements included
in this press release are expressly qualified in their entirety by the
foregoing cautionary statements. You are cautioned not to place undue
reliance on these forward-looking statements, which speak only as of
today's date. We do not undertake any obligation to update, amend or
clarify these statements or the "Risk Factors" contained in our most
recent reports filed with the SEC, whether as a result of new
information, future events or otherwise, except as may be required under
the applicable securities laws.

View source version on businesswire.com: http://www.businesswire.com/news/home/20160526005720/en/
Source: Masimo
Masimo
Irene Paigah, 858-859-7001
irenep@masimo.com